HOW IS ALCOHOL MADE
The alcoholic drinks we drink is a chemical called ethanol
ETHANOL PRODUCTION PROCESS
The type of alcohol in the alcoholic drinks we drink is a chemical called ethanol. For ethanol production, you need to put grains, fruits or vegetables through a process called fermentation (when yeast or bacteria react with the sugars in food – the by-products are ethanol and carbon dioxide).
FERMENTATION
Wine and cider are made by fermenting fruit, while fermented cereals such as barley and rye form the basis of beer and spirits.
A drink’s alcohol content is affected by how long it’s left to ferment.

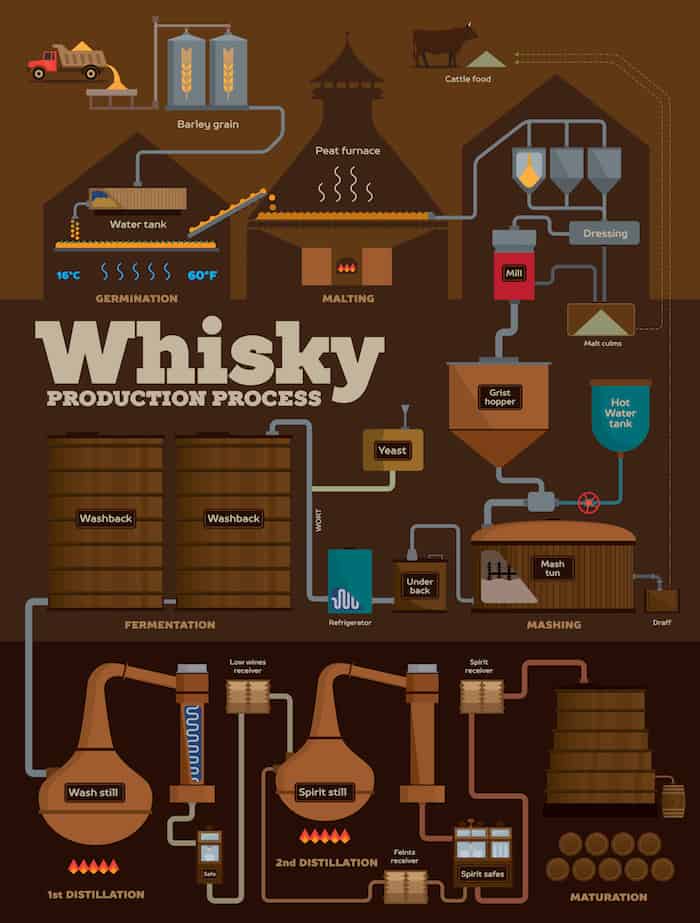
DISTILLATION
Ethanol production of distilled spirits is based upon fermentation, the natural process of decomposition of organic materials containing carbohydrates. It occurs in nature whenever the two necessary ingredients, carbohydrate and yeast, are available. Yeast is a vegetative microorganism that lives and multiplies in media containing carbohydrates—particularly simple sugars. It has been found throughout the world, including frozen areas and deserts.
Distilled spirits are all alcoholic beverages in which the concentration of ethyl alcohol has been increased above that of the original fermented mixture by a method called distillation. The principle of alcoholic distillation is based upon the different boiling points of alcohol (78.5 °C, or 173.3 °F) and water (100 °C, or 212 °F). If a liquid containing ethyl alcohol is heated to a temperature above 78.5 °C but below 100 °C and the vapour coming off the liquid is condensed, the condensate will have a higher alcohol concentration, or strength.
Distilled spirit, also called distilled liquor, alcoholic beverage (such as brandy, whisky, rum, or arrack) that is obtained by distillation from wine or other fermented fruit or plant juice or from a starchy material (such as various grains) that has first been brewed. The alcoholic content of distilled liquor is higher than that of beer or wine.
MATURATION
Maturation provides whisky with a mild and smooth texture by removing the irritating alcoholic flavor. However, the precise mechanism by which the whisky flavor is improved through the maturation process remains unknown. In this study, we performed mesoscopic structural measurements—dynamic light scattering (DLS) and small‐angle X‐ray scattering (SAXS)—to elucidate the relationship between the liquid structure and flavor maturation of whiskies. Both techniques detected two scattering components corresponding to the clusters formed by the extractives from oak casks during maturation, which are not present in the new make (freshly distilled whisky). Analyzing the scattering profiles revealed that only the small clusters increase in concentration during maturation. It is concluded the small cluster component is crucial for obtaining flavorful whiskies, while the large cluster component, whose concentration is independent of the maturation time, is related to the alcoholic irritation of the whiskies, as demonstrated by the sonication test.
Keywords: clusters, dynamic light scattering, maturation, small angle X‐ray scattering, whisky
WHY IS IT ILLEGAL TO HAVE YOUR OWN STILL?
Unless the thought of digging ditches with chains around your ankles is appealing, you better read this article on the legalities of making moonshine very closely. There are federal and state laws regulating home distilling. To avoid trouble with the federal, state, and local law enforcement agencies and officials, you’ll need to comply with federal and state laws, as well as any local laws. We’ll summarize federal laws in this article, which are important to know. However, also be sure to check out laws regulating distillation for your state.
Keywords: clusters, dynamic light scattering, maturation, small angle X‐ray scattering, whisky

